- Synonyms
- Cellular Thyroid Hormone-binding Protein, Prolyl 4-hydroxylase Subunit beta, p55
- Source
- Escherichia coli.
- Molecular Weight
- Approximately 56.6 kDa, a single non-glycosylated polypeptide chain containing 502 amino acids. (MRGSGSHHHHHH-PDI).
- AA Sequence
- MRGSGSHHHH HHAPEEEDHV LVLRKSNFAE ALAAHKYLLV EFYAPWCGHC KALAPEYAKA AGKLKAEGSE IRLAKVDATE ESDLAQQYGV RGYPTIKFFR NGDTASPKEY TAGREADDIV NWLKKRTGPA ATTLPDGAAA ESLVESSEVA VIGFFKDVES DSAKQFLQAA EAIDDIPFGI TSNSDVFSKY QLDKDGVVLF KKFDEGRNNF EGEVTKENLL DFIKHNQLPL VIEFTEQTAP KIFGGEIKTH ILLFLPKSVS DYDGKLSNFK TAAESFKGKI LFIFIDSDHT DNQRILEFFG LKKEECPAVR LITLEEEMTK YKPESEELTA ERITEFCHRF LEGKIKPHLM SQELPEDWDK QPVKVLVGKN FEDVAFDEKK NVFVEFYAPW CGHCKQLAPI WDKLGETYKD HENIVIAKMD STANEVEAVK VHSFPTLKFF PASADRTVID YNGERTLDGF KKFLESGGQD GAGDDDDLED LEEAEEPDME EDDDQKAVKD EL
- Purity
- > 95 % by SDS-PAGE and HPLC analyses.
- Thiol proteinReductase activity
- Thiol Protein Reductase Activity is 0.001 Δ650nm/ min-2, determined by measuring the turbidity increase at 650 nm due to insulin reduction. The activity is expressed as the ratio of the slope of a linear part of the turbidity curve to the lag time.
- Isomerase activity
- Isomerase Activity is 0.5 µmol active RNase A min-1 µmol PDI-1, according to the re-activation of reduced and denatured RNase A.
- Physical Appearance
- Sterile Filtered White lyophilized (freeze-dried) powder.
- Formulation
- Lyophilized from a 0.2 µm filtered concentrated solution in PBS, pH 7.0.
- Endotoxin
- Less than 1 EU/µg of rHuPDI as determined by LAL method.
- Reconstitution
- We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/ml. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions.
- Stability & Storage
- Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 3 months, -20 to -70 °C under sterile conditions after reconstitution.
- Usage
- This material is offered by Shanghai PrimeGene Bio-Tech for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE.
- SDS-PAGE
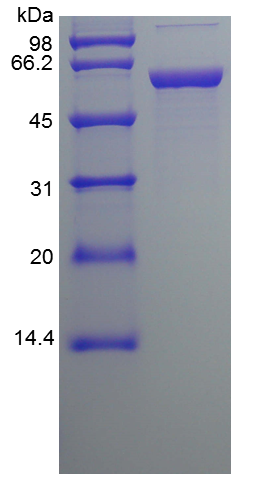
- Reference
- 1. Pihlajaniemi, T., T. Helaakoski, K. Tasanen, et al. 1987. EMBO J, 6: 643-9.
2. Tasanen, K., T. Parkkonen, L.T. Chow, et al. 1988. J Biol Chem, 263: 16218-24.
3. Wilkinson, B., and H.F. Gilbert. 2004. Biochim Biophys Acta, 1699: 35-44.
- Background
- Protein disulfide isomerases (PDIs) constitute a family of structurally related enzymes which catalyze disulfide bonds formation, reduction, or isomerization of newly synthesized proteins in the lumen of the endoplasmic reticulum (ER). They act also as chaperones, and are, therefore, part of a quality-control system for the correct folding of the proteins in the same subcellular compartment. PDI has been found to have moderate effects (25-fold) on the rate of oxidative folding of proteins in vitro.�Recombinant Human Protein Disulfide Isomerase is involved in disulphide-bond formation and isomerization, as well as the reduction of disulphide bonds in proteins. Recombinant PDI has been found to have moderate effects (25-fold) on the rate of oxidative folding of proteins in vitro.

 COA申请
COA申请
