- Synonyms
- SOD1
- Source
- Escherichia coli.
- Molecular Weight
- Approximately 31.6 kDa, a homodimer, non-glycosylated polypeptide chain containing 2 × 153 amino acids.
- AA Sequence
- ATKAVCVLKG DGPVQGIINF EQKESNGPVK VWGSIKGLTE GLHGFHVHEF GDNTAGCTSA GPHFNPLSRK HGGPKDEERH VGDLGNVTAD KDGVADVSIE DSVISLSGDH CIIGRTLVVH EKADDLGKGG NEESTKTGNA GSRLACGVIG IAQ
- Purity
- > 95 % by SDS-PAGE and HPLC analyses.
- Biological Activity
- Fully biologically active when compared to standard. The potency per mg was determined by pyrogallol autoxidation method and was found to be more than 3000 U/mg.
- Physical Appearance
- Sterile Filtered White lyophilized (freeze-dried) powder.
- Formulation
- Lyophilized from a 0.2 μm filtered concentrated solution in PBS, pH 7.4.
- Endotoxin
- Less than 1 EU/μg of rHuCu/Zn SOD as determined by LAL method.
- Reconstitution
- We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions.
- Stability & Storage
- Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 3 months, -20 to -70 °C under sterile conditions after reconstitution.
- Usage
- This material is offered by Shanghai PrimeGene Bio-Tech for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE.
- SDS-PAGE
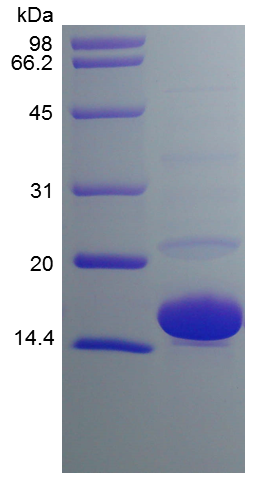
- Reference
- 1. Kwiatowski J, Skarecky D, Ayala FJ. 1992. Mol Phylogenet Evol, 1: 72-82.
2. Bachus R, Claus A, Megow D, et al. 1995. J Neurol Sci, 129 Suppl: 93-5.
3. Qi X, Guy J, Nick H, et al. 1997. Invest Ophthalmol Vis Sci, 38: 1203-12.
4. Chou CM, Huang CJ, Shih CM, et al. 2005. Ann N Y Acad Sci, 1042: 303-13.
5. Raja SB, Murali MR, Roopa K, et al. 2011. Biomed Pharmacother, 65: 560-8.
- Background
- Superoxide dismutase catalyzes the reaction between superoxide anions and hydrogen to yield molecular oxygen and hydrogen peroxide. Cu/Zn superoxide dismutase also named as SOD1, is an enzyme encoded by the SOD1 gene in humans, located on chromosome 21. The SOD1 binds Cu and Zn ions and is one of three SODs responsible for destroying free superoxide radicals in the body. It has been shown to interact with CCS and Bcl-2. The malfunction of SOD1 may increase the risk of illnesses like age-related muscle mass loss (sarcopenia), early development of cataracts, macular degeneration, thymic involution, hepatocellular carcinoma, shortened lifespan, keratoconus and amyotrophic lateral sclerosis.







 COA申请
COA申请
