- Synonyms
-
- Source
- Escherichia coli.
- Molecular Weight
- Approximately 19.4 kDa, a single non-glycosylated polypeptide chain containing 166 amino acids.
- AA Sequence
- MCDLPQTHSL GSRRTLMLLA QMRRISLFSC LKDRHDFGFP QEEFGNQFQK AETIPVLHEM IQQIFNLFST KDSSAAWDET LLDKFYTELY QQLNDLEACV IQGVGVTETP LMKEDSILAV RKYFQRITLY LKEKKYSPCA WEVVRAEIMR SFSLSTNLQE SLRSKE
- Purity
- > 96 % by SDS-PAGE and HPLC analyses.
- Biological Activity
- Fully biologically active when compared to standard. The specific activity determined by an anti-viral assay is no less than 1.0 × 108 IU/mg.
- Physical Appearance
- Sterile Filtered White lyophilized (freeze-dried) powder.
- Formulation
- Lyophilized from a 0.2 µm filtered solution in PBS, pH 7.4.
- Endotoxin
- Less than 1 EU/µg of rHuIFN-α2b, Ecoli. as determined by LAL method.
- Reconstitution
- We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Reconstitute in sterile distilled water or aqueous buffer containing 0.1 % BSA to a concentration of 0.1-1.0 mg/mL. Stock solutions should be apportioned into working aliquots and stored at ≤ -20 °C. Further dilutions should be made in appropriate buffered solutions.
- Stability & Storage
- Use a manual defrost freezer and avoid repeated freeze-thaw cycles.
- 12 months from date of receipt, -20 to -70 °C as supplied.
- 1 month, 2 to 8 °C under sterile conditions after reconstitution.
- 3 months, -20 to -70 °C under sterile conditions after reconstitution.
- Usage
- This material is offered by Shanghai PrimeGene Bio-Tech for research, laboratory or further evaluation purposes. NOT FOR HUMAN USE.
- SDS-PAGE
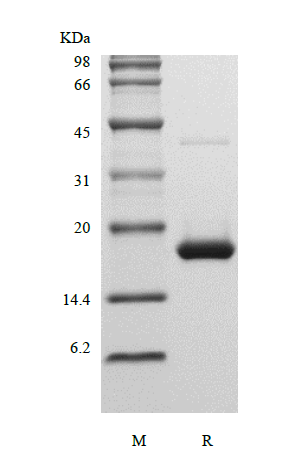
- Reference
- 1. Tarhini AA, Gogas H, Kirkwood JM. 2012. J Immunol, 189: 3789-93.
2. Tohyama M, Yang L, Hanakawa Y, et al. 2012. J Invest Dermatol, 132: 1933-5.
3. Corssmit EP, Heijligenberg R, Hack CE, et al. 1997. Clin Exp Immunol, 107: 359-63.
4. Corssmit EP, de Metz J, Sauerwein HP, et al. 2000. J Interferon Cytokine Res, 20: 1039-47.
- Background
- IFN-αs are proteins secreted by leukocyte. They are mainly involved in innate immune response against viral infection. The IFN-α family has 13 subtypes and 23 different variants. The individual proteins have molecular masses between 19-26 kDa and consist of proteins with lengths of 156-166 and 172 amino acids. All IFN-α subtypes possess a common conserved sequence region between amino acid positions 115-151 while the amino-terminal ends are variable. Many IFN-alpha subtypes differ in their sequences at only one or two positions. Naturally occurring variants also include proteins truncated by 10 amino acids at the carboxy-terminal end.







 COA申请
COA申请
